
Note the usefulness of the periodic table in predicting likely ion formation and charge ( Figure 2.29). (A discussion of the theory supporting the favored status of noble gas electron numbers reflected in these predictive rules for ion formation is provided in a later chapter of this text.) It has the same number of electrons as atoms of the next noble gas, krypton, and is symbolized Br −. This results in an anion with 35 protons, 36 electrons, and a 1− charge.

For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 17 gain one electron and form anions with a 1− charge atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. The name of a metal ion is the same as the name of the metal atom from which it forms, so Ca 2+ is called a calcium ion.
It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized Ca 2+. This results in a cation with 20 protons, 18 electrons, and a 2+ charge. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion.
Carbon tetrachloride formula ionic or molecular plus#
(b) A sodium cation (Na +) has lost an electron, so it has one more proton (11) than electrons (10), giving it an overall positive charge, signified by a superscripted plus sign. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say “monoxide” rather than “monooxide” and “trioxide” rather than “troxide”).Figure 2.28 (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged.
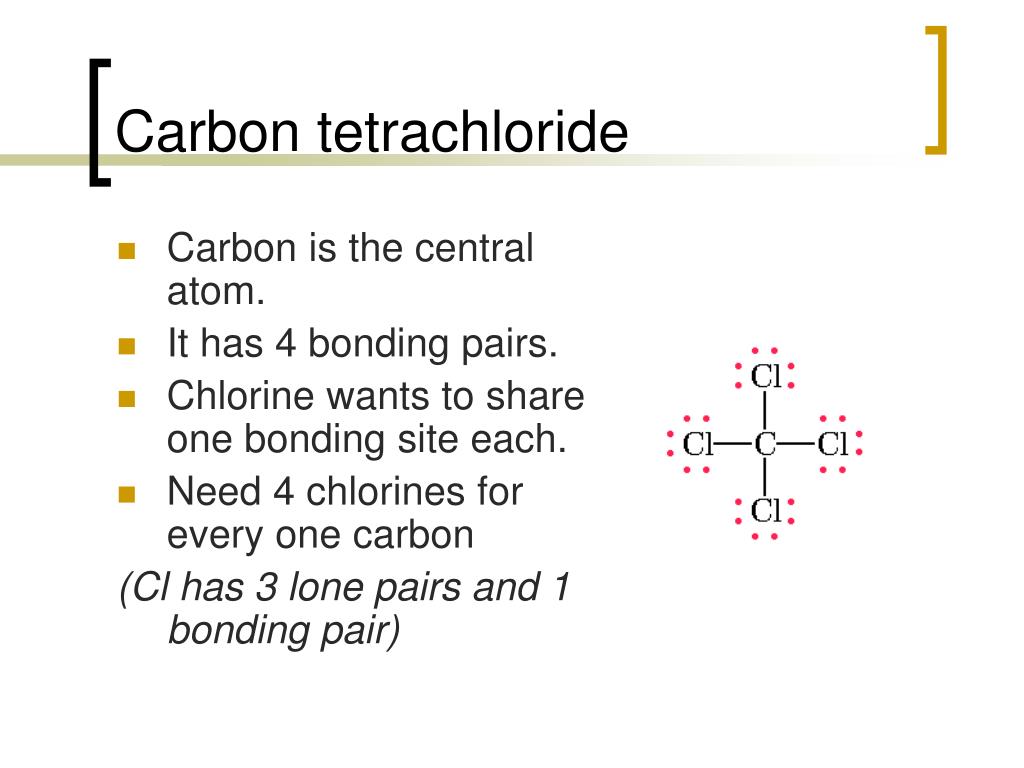
Normally, no prefix is added to the first element’s name if there is only one atom of the first element in a molecule. Table 4.1 “Numerical Prefixes for Naming Binary Covalent Compounds” lists these numerical prefixes. A system of numerical prefixes is used to specify the number of atoms in a molecule.

The second element is named by taking the stem of the element name and adding the suffix – ide. The first element in the formula is simply listed using the name of the element. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. For example, we have already seen CH 4, the molecular formula for methane. Numerical subscripts are used if there is more than one of a particular atom. Then the other nonmetal symbols are listed. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H 2O is the prominent exception). The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules.


 0 kommentar(er)
0 kommentar(er)
